Gonal-f is a hormonal drug used in assisted reproduction treatments to cause the development of multiple follicles in the ovary.
It is composed of follicle-stimulating hormone (FSH), a gonadotropin that acts on a woman's menstrual cycle.
There are several formats of Gonal-f on the market, but one of the best known is that administered with a pen type injector.
Provided below is an index with the 6 points we are going to expand on in this article.
Composition and packaging
The active ingredient of Gonal-f is alpha-folithropin, a recombinant FSH obtained by genetic engineering from Chinese hamster ovary cells.
The Gonal-f pen format consists of a pre-filled multi-dose pen that provides different amounts of the medication depending on the dose purchased at the pharmacy.
Specifically, Gonal-f pen is marketed for these 3 doses:
- Gonal-f 300
- pen with 300 IU in 0.5 milliliters, which is equivalent to 22 micrograms of FSHr.
- Gonal-f 450
- pen with 450 IU in 0.75 milliliters, which is equivalent to 33 micrograms of FSHr.
- Gonal-f 900
- pen with 900 IU in 1.5 milliliters, which is equivalent to 66 micrograms of FSHr.
Depending on the dosage set by the gynecologist within each treatment, it will be necessary to buy one type of Gonal-f or another. Normally, in in vitro fertilization (IVF) treatments, the Gonal-f 900 is usually purchased, since greater stimulation of the ovaries is needed.
The costs of the Gonal-f pen are as follows: Gonal-f 300 costs $160,00 Gonal-f 450 costs $260,00 and Gonal-f 900 costs $490,00.
Considering seeing a fertility specialist? Don't forget that, in the field of Reproductive Medicine, as in any other medical area, it is crucial that patients rely on the doctors and staff that will help them through their treatment cycle. Logically, conditions vary from clinic to clinic. For this reason, we recommend that you generate your Fertility Report now. It will offer you a list of clinics that have passed our rigorous selection process successfully. Furthermore, the system will make a comparison between the fees and conditions of each clinic so that you can make a better-informed decision.
Mode of administration
Medications used for controlled ovarian stimulation are started at the beginning of the menstrual cycle, once a woman's period begins. Normally, Gonal-f begins to be injected on the second or third day.
It is very important to always follow your doctor's instructions for administering this drug and to do so at the same time every day.
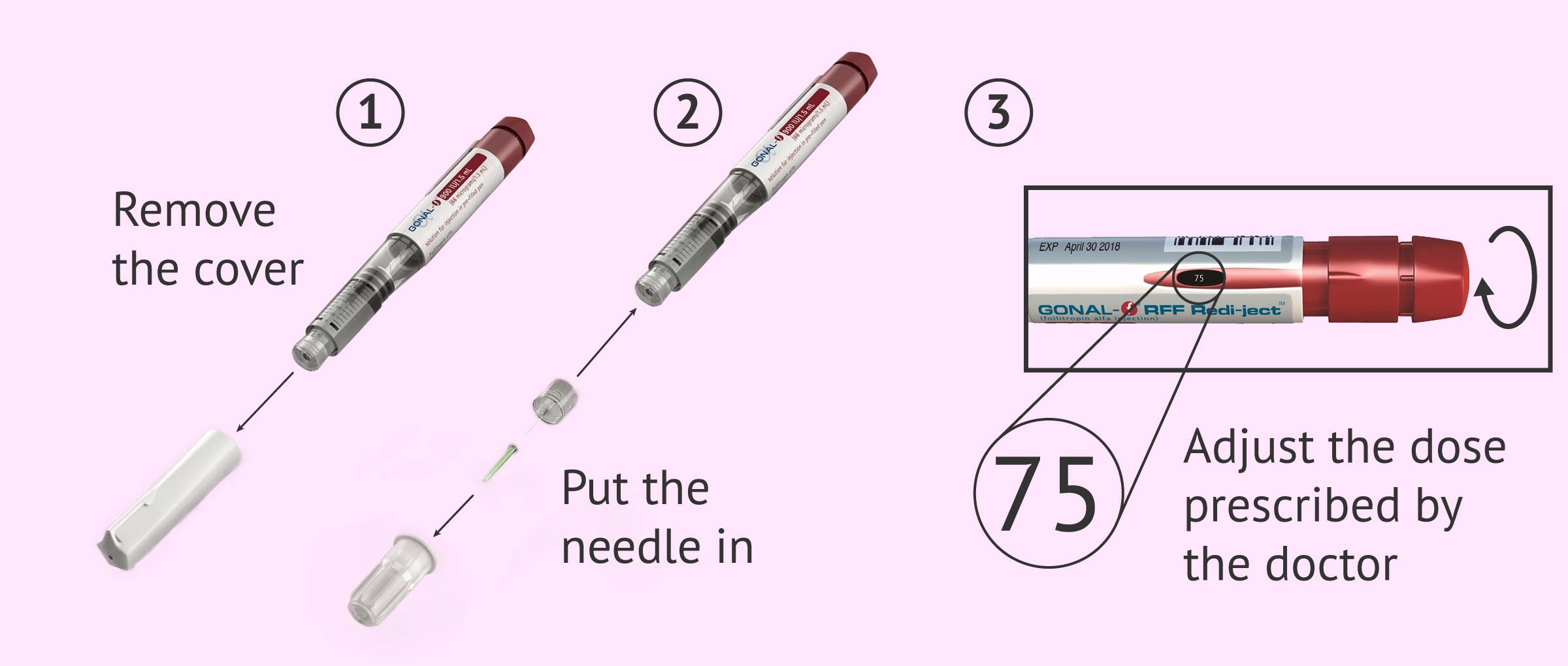
The Gonal-f pre-filled pen comes with several needles that can be attached, so the Gonal-f pen can be used for several injections.
Administration is subcutaneous, just under the skin, in the abdomen or on the thighs. In the following section we are going to to detailed instructions for use:
- Always put a new needle in the pen before injecting.
- Check that there is a drop of liquid on the tip of the needle.
- Set the dose of Gonal-f prescribed by the doctor: Turn the dose setting knob until the desired dose appears in the information window.
- Choosing the injection site. It is advisable to change the injection site every day to reduce irritation.
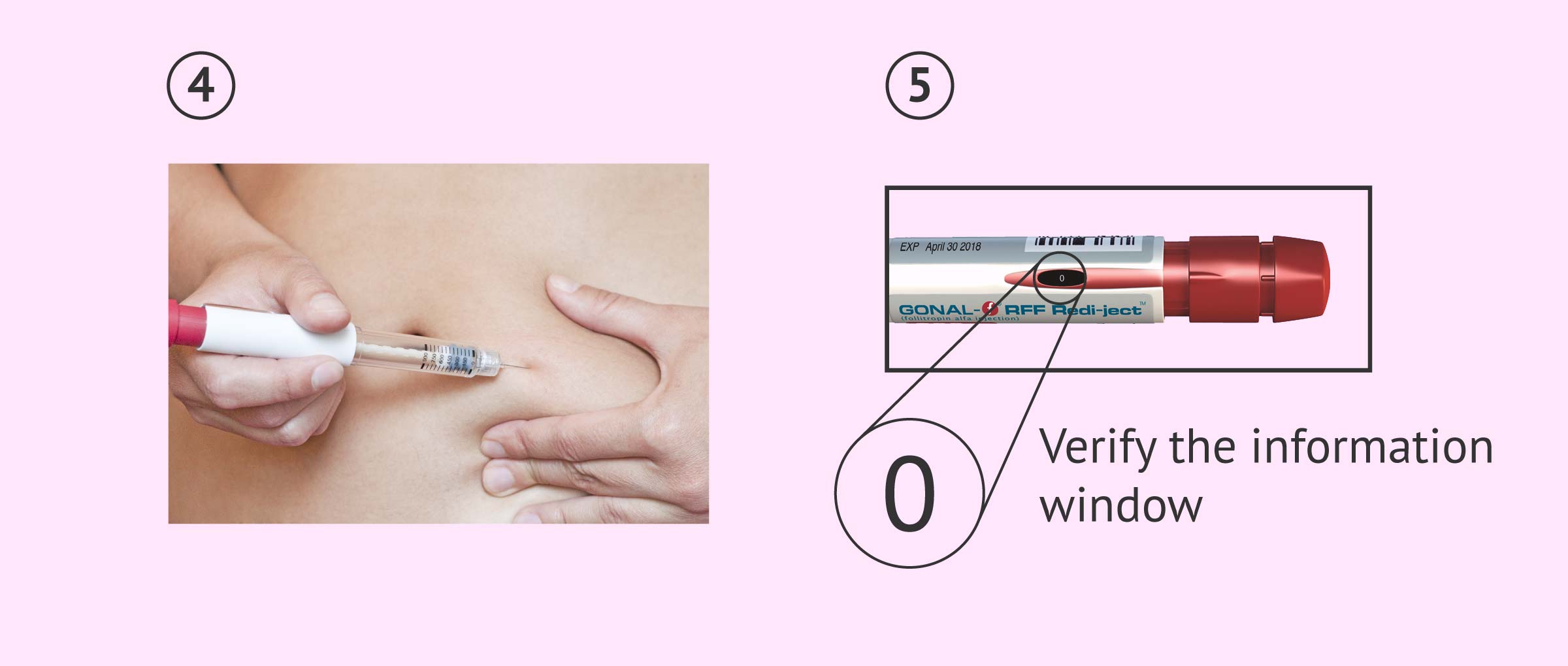
- Slowly insert the entire needle into the skin and press the dose setting button fully for about 5 seconds.
- Remove the needle from the skin by keeping the dose setting button pressed.
- Check in the dose information window that the injection has been completed. If not, use a new pen to administer the missing dose, which will appear in the information window.
- Remove the needle with the help of the external cover and do not use it again.
The Gonal-f pen comes with a treatment diary that is very useful for recording the amount of IU administered each day and the time of injection. This way, it is easier to control how many injections you can make with each pen depending on your dose and whether you need to buy a new pen.
FAQs from users
How should the Gonal-f pen be preserved?
It is recommended that the Gonal-f pen be stored in the refrigerator, at a temperature between 35°F and 46°F, without freezing; although it is also possible to store it outside the refrigerator, at a temperature below 77°F, for a maximum of 28 days once opened.
It is also important to keep this drug in its original packaging to protect it from light and never store it with the needle in it.
What is the purpose of the Gonal-f pen?
Gonal-f is a hormonal drug that serves to stimulate a woman's ovaries and trigger the development of follicles during an artificial insemination, conventional IVF or ICSI cycle.
On some occasions, Gonal-f is also prescribed to men to stimulate sperm production, a process known as spermatogenesis.
What happens if I miss a day of administering the dose of Gonal-f?
It is not advisable to inject a double dose of Gonal-f in case you forgot to take it the day before. It is best to contact your doctor as soon as possible and follow his instructions.
Suggested for you
Gonal-f is also marketed in other formats and different doses. If you want to get all the information about this medicine, we recommend you to read on in the next post: Gonal-f: dose, prices and administration for IVF.
We make a great effort to provide you with the highest quality information.
🙏 Please share this article if you liked it. 💜💜 You help us continue!
References
Agencia Española de Medicamentos y Productos Sanitarios. CIMA. GONAL-F 300 UI/0,5 ml (22 microgramos/0,5 ml) SOLUCION INYECTABLE EN PLUMA PRECARGADA. Nº REGISTRO: 95001033. Ficha técnica o resumen de las características del producto. (Link)
Agencia Española de Medicamentos y Productos Sanitarios. CIMA. GONAL-F 450 UI/0,75 ml (33 microgramos/0,75 ml) SOLUCION INYECTABLE EN PLUMA PRECARGADA. Nº REGISTRO: 95001034. Ficha técnica o resumen de las características del producto. (Link)
Agencia Española de Medicamentos y Productos Sanitarios. CIMA. GONAL-F 900 UI/1,5 ml (66 microgramos/1,5 ml) SOLUCION INYECTABLE EN PLUMA PRECARGADA. Nº REGISTRO: 95001035. Ficha técnica o resumen de las características del producto. (ver)
European Medicines Agency. GONAL-f: EPAR - Product Information. Última actualización: 10/08/2018 (Link)
European Medicines Agency. GONAL-f: EPAR - Summary for the public. Última actualización: 28/09/2010 (Link)
FAQs from users: 'How should the Gonal-f pen be preserved?', 'What is the purpose of the Gonal-f pen?' and 'What happens if I miss a day of administering the dose of Gonal-f?'.