Preimplantation genetic diagnosis (PGD) is a prevention technique used in assisted reproduction in order to detect abnormalities in the genetic material of embryos. It is, therefore, a complementary technique that will help to choose the best embryo to transfer in an in vitro fertilisation (IVF) treatment.
Thanks to PGD, it is possible to avoid the transfer of embryos with genetic or chromosomal alterations and, in this way, increase the probability of having a healthy child.
This technique can be applied to all patients who have undergone IVF treatment and obtained embryos. However, there are situations in which PGD is more recommended in order to obtain a better prognosis of the reproductive treatment.
Provided below is an index with the 10 points we are going to expand on in this article.
- 1.
- 2.
- 3.
- 4.
- 4.1.
- 4.2.
- 4.3.
- 4.4.
- 5.
- 6.
- 7.
- 7.1.
- 7.2.
- 7.3.
- 7.4.
- 7.5.
- 7.6.
- 7.7.
- 7.8.
- 7.9.
- 7.10.
- 7.11.
- 8.
- 9.
- 10.
What is PGD?
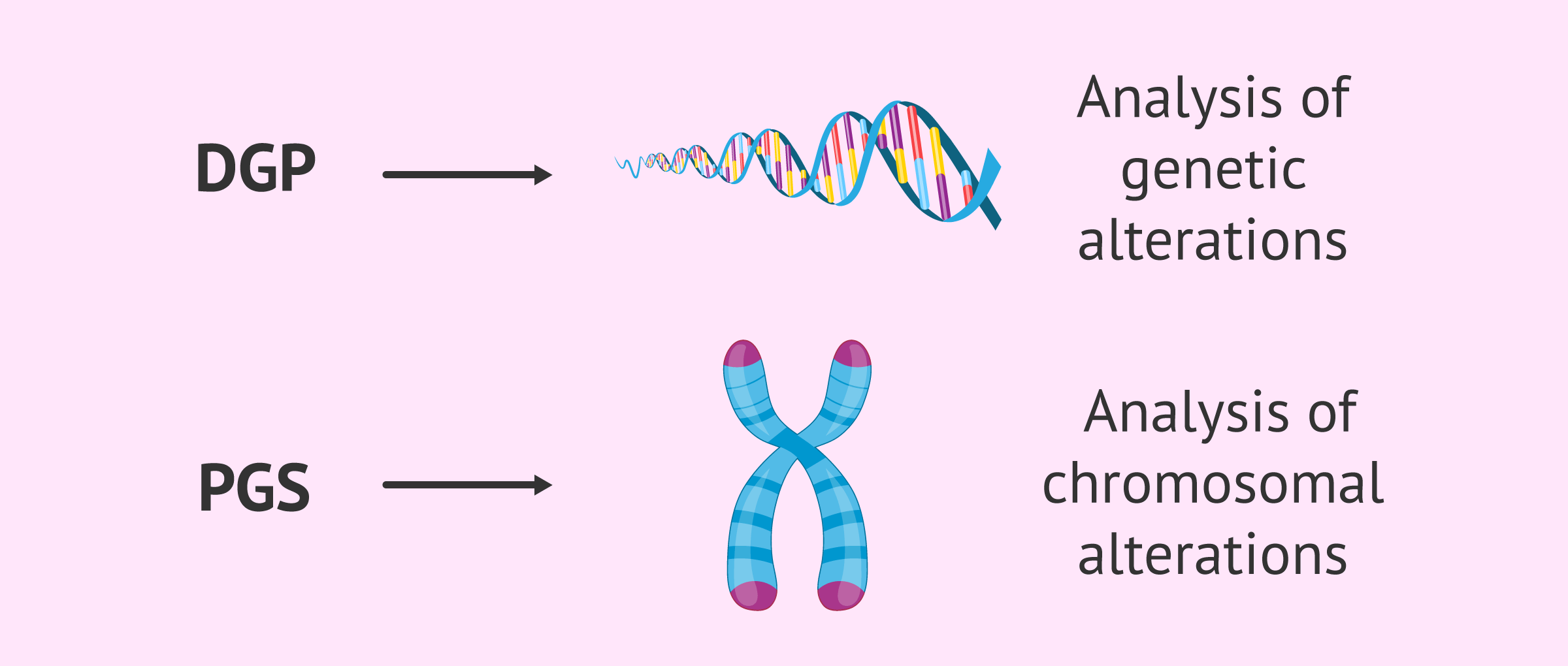
Although, in general, the term preimplantation genetic diagnosis or PGD is used to refer to the use of this technique, the truth is that two concepts are distinguished depending on the purpose :
- Preimplantation genetic diagnosis or PGD
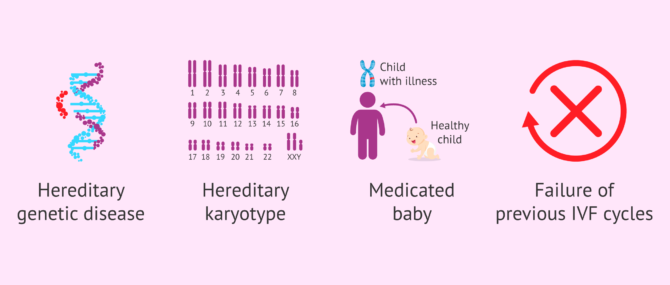
- allows the early detection of serious genetic diseases, which can be transmitted to offspring if the parents are carriers or sick. In general, these are monogenic hereditary diseases such as Fragile X syndrome, Huntington's disease and muscular dystrophy.
- Preimplantation genetic screening or PGS
- also called screening for aneuploidy. In this case, alterations in the number or structure of chromosomes are identified. The best known chromosomal disease is Down syndrome.
Depending on whether genetic or chromosomal alterations are to be detected, the techniques for analyzing the DNA of the embryos will be different.
When is PGD used?
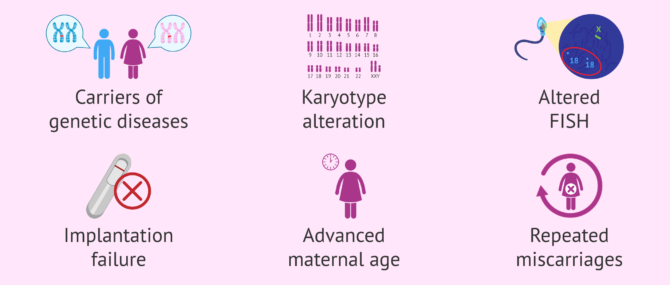
In general, PGD is an advantageous method, and for this reason, it is indicated in the following cases:
- When the intended parents, or at least one of them, are carriers of some hereditary genetic disease, and decide to visit a genetic specialist.
- When the intended parents, or at least one of them, have their karyotype altered (chromosomal testing) due to the presence of a chromosomal abnormality, such as translocations or chromosome inversions.
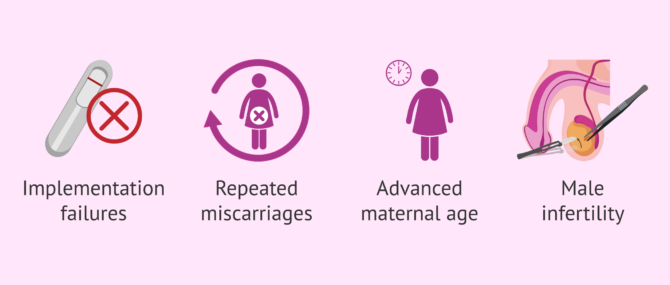
- After repeated conventional IVF or ICSI failure
- After recurrent embryo implantation failure (RIF)
- Recurrent pregnancy loss
- Advanced maternal age (especially indicated for women over 38-40 years old)
- History of fetal aneuploidy (abnormal number of chromosomes in a cell) in previous pregnancies
- Certain cases of male sterility, such as those when the semen sample is collected directly from the epididymis or the testis.
Like with any other treatment, the use of PGD has some legal limitations that vary from country to country. In Europe, for example, main IVF destinations such as Spain have restricted PGD to be applied only in the following cases:
- Prevention of severe, early-onset hereditary diseases for which there exists no postnatal cure.
- Prevention of abnormalities that may compromise the viability of the embryo.
- As a therapeutic method to heal or cure a sick child (savior baby or savior sibling): by selecting the human leukocyte antigens (HLA) of future offspring, the newborn can help cure a sibling's genetic disease.
To get detailed information about the disorders that can be detected through PGD, please do not miss the following article: Genetic diseases and PGD.
Procedure
In order to perform PGD on the embryos, the couple or woman must be undergoing IVF treatment. Therefore, the first step is to perform ovarian stimulation that allows obtaining a high number of eggs to fertilize.
After an ovarian puncture, the ovules are fertilized using the ICSI technique (intracytoplasmic sperm microinjection) to obtain embryos.
PGD can be done on both 3-day-old embryos in culture and 5-day-old blastocysts. In the latter case, it is possible to extract a greater number of cells from the trophectoderm to carry out the genetic study.
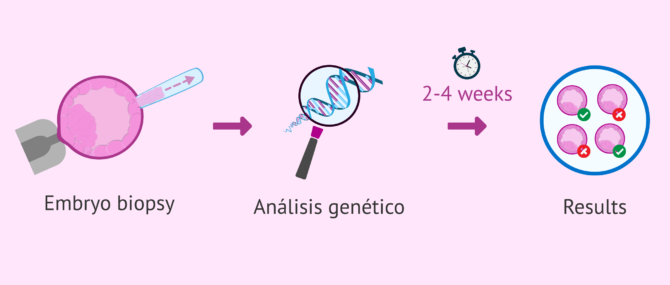
The steps to perform PGD in each of the embryos are as follows:
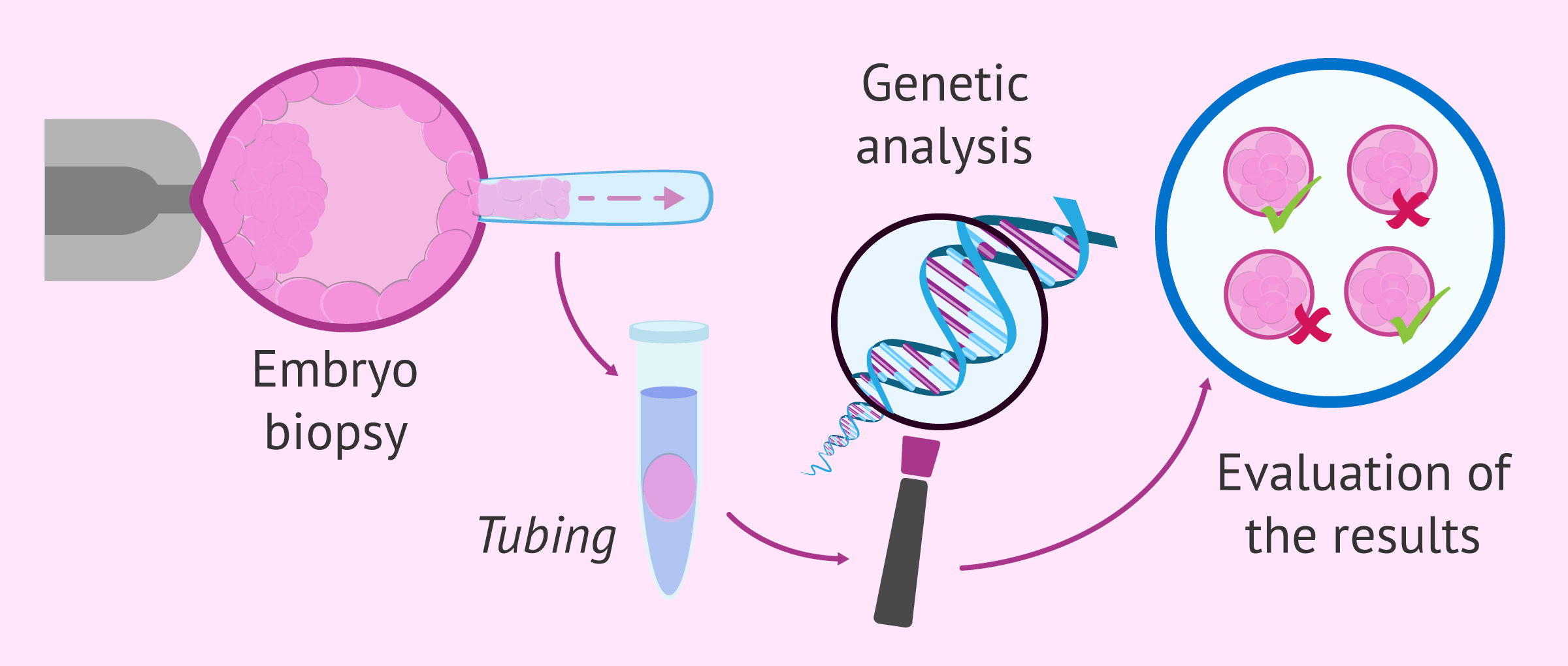
- Embryo biopsy: a hole is made by means of a laser or chemical substances in the zona pellucida of the embryo to extract one or two cells, if the embryo is 3 days old, or several cells of the trophectoderm in the case of blastocysts.
- Tubing or intubated: the extracted cells are placed in a tube with great delicacy. Subsequently, the genetic material that each cell contains inside will be extracted.
- Analysis of the extracted DNA: there are several techniques such as FISH, array CGH, PCR, or sequencing.
- Assessment of results: genetically healthy embryos and those with alterations in their genetic material are identified, which will be discarded.
Lastly, the quality of the embryos that are genetically viable is assessed in order to transfer them to the mother's uterus. On the other hand, the remaining embryos will be vitrified for future attempts.
For further information, we recommend you to have a look at the following post: About the PGD process.
What are the pros and cons?
PGD applied to the prevention of serious hereditary diseases has a clear benefit, as it avoids the couple having to decide whether or not to terminate the pregnancy because they are carrying a sick baby.
On the other hand, PGD to treat infertility and increase success rates does give rise to debate between proponents and opponents of the technique because of the ethical considerations involved.
In the following, we will discuss the advantages and disadvantages of PGD, as well as some ethical and legal aspects.
Benefits of PGD
People who decide to do a PGD while trying to get pregnant can achieve the following benefits:
- Best embryo selection
- Obviously, the main objective of PGD is to detect genetically healthy embryos. Therefore, those with mutations or aneuploidies that, without this genetic analysis, could be confused and transferred to the mother, which would lead to implantation failure, miscarriage, or birth of a sick child, can be directly ruled out.
- Lower risk of abortions
- some chromosomal alterations allow the embryo to implant, but after several weeks of development they end up in a spontaneous abortion due to not having the correct genetic makeup.
- Higher pregnancy rate
- since embryos that lead to repeated implantation failures are avoided.
- Less number of IVF treatments
- PGD allows "getting it right" in the selection of the embryo with greater implantation capacity and that can give rise to a healthy baby. Therefore, the number of failed transfers is reduced, as is the time to achieve a pregnancy.
- Greater peace of mind for patients
- PGD manages to eliminate the uncertainty of whether the embryo will be good or not. In addition, once the positive is obtained, the woman also feels more relaxed knowing that the embryo is viable and the risk of miscarriage is much lower.
As previously mentioned, applying the PGD technique reduces the number of IVF treatments. In addition, this also has the advantage of reducing the financial cost involved. It is true that PGD means having to pay an additional amount of money, but it is also possible that pregnancy may occur earlier than if the genetic analysis were not applied. Therefore, in this case, the cost of future embryo transfers would be reduced or avoided.
DGP drawbacks
The application of PGD also has, like the rest of assisted reproduction techniques, some drawbacks:
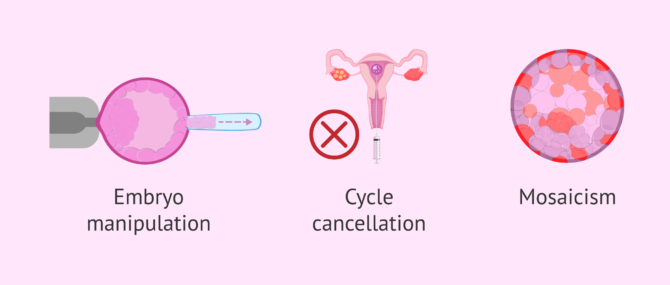
- Handling the embryo
- embryo biopsy is an invasive procedure that involves making a hole in the zona pellucida and, furthermore, allowing it to spend more time outside the incubator. Some embryos do not support this process and stop their development.
- Cancellation of the cycle
- PGD implies having to discard several embryos due to the anomalous result. If the couple did not have many embryos after fertilization, the risk of having to cancel the transfer is greater.
- Mosaicism
- some embryos present a mixture of normal cells and altered cells. Therefore, when only one cell of the embryo is biopsied, it is possible that a non-viable mosaic embryo is taken as healthy, or vice versa.
The remaining drawbacks of PGD have to do with ethical and moral issues, which will be discussed in the next section.
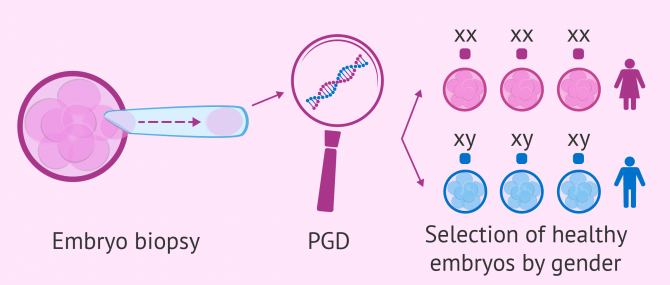
PGD for gender selection
Using PGD as a method for gender selection is subject to a number of legal restrictions and controversy, and for this reason, it is not allowed everywhere across the world.
Currently, sex selection is permitted in the UK, but only in cases where a genetic disease is linked to one sex rather than the other, such as Duchenne muscular dystrophy, a disease that affects males.
When done for medical reasons, the embryo is tested to find out its sex. Then, only the embryos of the non-affected sex are selected for the transfer. This way, we prevent children from having serious medical conditions.
The process of PGD for gender selection works as follows:
- Patients undergo normal IVF treatment to create the embryos
- Embryos are cultured in the laboratory for a number of days
- Some cells are removed from the embryo(s) selected
- The chromosomes are examined to identify which ones are male and which are female
- The embryos of the desired gender are transferred to the patient's uterus
In the United States, the regulations governing PGD allow gender selection for social reasons (a phenomenon known by many as "designer babies"), and this is why it is the main destination for fertility tourists from the UK, Australia, and Canada above all.
The unused embryos of the appropriate sex can be cryopreserved (embryo freezing) for later use, although not all embryos biopsied may be suitable for vitrification. Those of the different sex can be destroyed or donated to science.
It should be clear that this test is not 100% reliable. Also, there is the risk that no embryo is suitable for transfer because all embryos obtained are of the opposite gender.
Ethical issues and controversy
With PGD, some embryos are considered "viable", i.e. the healthy ones, while others are ruled out and considered to be "non-viable", as they carry a genetic abnormality. This has created a debate in which opponents ask themselves to what extent is it respectful of the morality and ethics of the process. The main arguments are:
- How ethical is it to select an embryo in vitro so that it becomes a savior baby once born?
- Is it ethical to choose the gender of your baby for social purposes (physical features such as eye color)?
- To what extent am I acting in an ethical way if I select only the viable embryos and allow the affected ones to perish?
- If a couple undergoes IVF, do they have the right to find out the genome of their embryos?
- How ethical is it to use PGD in the case of late-onset diseases?
- Where should the limits to embryo selection by genetic testing be set?
The controversy surrounding this technique is precisely the reason why it has not been legalized in a number of countries. If allowed, the law contemplates a number of legal restrictions as well.
What is the cost of PGD?
Preimplantation genetic diagnosis is an additional complexity to the usual process of in vitro fertilisation, so the economic cost is higher.
PGD may add up to €3,000-4,000 to the process of IVF with ICSI. These additional fees depend on whether one wishes to have only the most common chromosomes analyzed by FISH (13, 15, 16, 17, 18, 21, 22, X, and Y), the common ones with some additional chromosome, or all of them.
It also varies depending on factors such as advanced female age, the method used for genetic testing, etc.
Assisted procreation, as any other medical treatment, requires that you rely on the professionalism of the doctors and staff of the clinic you choose. Obviously, each clinic is different. Get now your Fertility Report, which will select several clinics for you out of the pool of clinics that meet our strict quality criteria. Moreover, it will offer you a comparison between the fees and conditions each clinic offers in order for you to make a well informed choice.
Patients are recommended to ask fertility clinics for providing them with a detailed cost estimate including all fees so that couples are able to see the price of embryo biopsy, standard genetic screening, and all tests done as complementary testing.
Even though the overall cost can vary from clinic to clinic, in general, the cost of IVF-ICSI with PGD ranges from €8,000 to €9,000.
Video on the probability of success of IVF with PGD
In the following video, Dr. Jon Díez Alcántara, gynaecologist at Fertility Madrid, talks about PGD and its chances of success.
The main benefit we have with the PDG is knowing in advance which embryo is correct and avoid and we avoid transferring the bad ones.
FAQs from users
Is it possible to perform PGD on eggs?
At present, it is not possible to perform PGD on eggs. This technique would damage the eggs and it would not be possible to fertilize them to obtain embryos.
Read more
How long do PGD results take?
The time in which the results of the PGD are provided is usually between 2 and 4 weeks, although these may vary depending on the technique, the genetic laboratory, etc. Also, some laboratories offer the possibility of making a diagnosis in 24 hours, although these cases are usually studied, both because of the costs and the associated risks.
An important aspect is the case in which a previous genetic study is required to analyze hereditary diseases. These cases involve a variable prior study depending on the pathology and knowledge of the disease that can take up to 2 months. After this period of time, the assisted reproduction treatment must be carried out, which will conclude with the genetic diagnosis. In other words, this process can take up to 4 months.
To what extent is the application of PGD important in women who decide to become mothers at an advanced age?
From a reproductive point of view, if we take as advanced maternal age women seeking pregnancy from 40-41 years of age, the clinical data are clear and reveal that the eggs of these women present an increased risk of chromosomal alterations, especially trisomies such as chromosome 21 or Down's Syndrome.
Clinical data from the main medical groups that apply PGD show that its use for this group of women favors the gestation rate and decreases the miscarriage rate.
What is the present and future of the DGP?
Today, PGD has become an intrinsic part of reproductive medicine and adds to the preventive options offered to couples with a personal or family history of severe hereditary diseases. It is also useful as a tool for improving reproductive options in specific groups of couples with subfertility or increased risk for chromosomally altered embryos.
The future of PGD seeks to integrate new knowledge and developments in high-throughput genetic methodologies, such as next-generation ultrasequencing platforms, together with advances in assisted reproductive techniques (ART) to improve reproductive options for all couples attending assisted reproduction clinics.
When is PGD recommended?
Answer by Crea Centro médico de Reproducción Asistida:
The PGD technique is indicated in the following cases:
- Couples who are carriers of a genetic disease, such as cystic fibrosis or osteogenesis imperfecta
- Alterations in the karyotype of one of the members of the couple
- Men with FISH in altered spermatozoa or severe male factor
- Implantation failures in IVF
- Advanced maternal age
- Repeat abortions
In the case of couples carrying a genetic alteration or monogenic disease, a study is needed prior to PGD to locate the mutation and be able to look for it in the cells of the embryo to be analyzed
What are the main risks of PGD?
As explained above, we put the embryo at risk of being seriously damaged after the biopsy to the point that the embryo transfer has to be cancelled, even though it was a healthy embryo. Also, the result of the genetic screening might show that all embryos are genetically altered, in which case the transfer would be cancelled as well, and a new IVF-ICSI cycle restarted. Moving to donor eggs and/or sperm is another feasible option.
Why is PGD indicated for women 40 years of age or older?
The eggs of these women are no longer of good quality and have an increased risk of chromosomal abnormalities. Therefore, PGD can increase the pregnancy rate, decrease the miscarriage rate and prevent the baby from having a genetic disease such as Down syndrome.
Is sex selection possible with PGD?
Yes, since the sex chromosomes are analyzed for any alteration, it is possible to know if the embryo is male or female. This is very useful in case the couple presents some genetic disease linked to sex, since only those embryos of one of the sexes, which are healthy, could be chosen for transfer.
How many embryos are necessary for PGD?
There is no minimum number of embryos for PGD. However, as it is an expensive procedure, couples who have obtained a low amount of embryos in a single cycle are advised to undergo more cycles in order for a higher number of embryos to be gathered (normally 5 or over) before PGD.
Is there any alternative to PGD to prevent the transmission of genetic diseases?
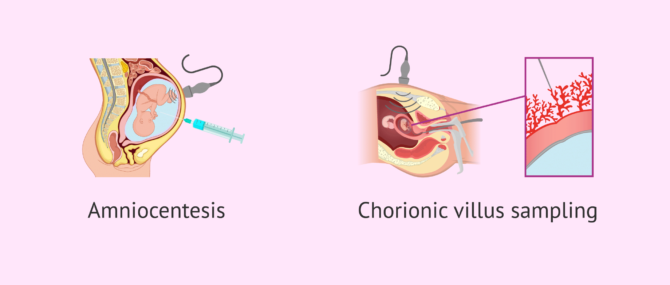
If preimplantation genetic diagnosis is not done, the only option left would be fetal or prenatal resting through amniocentesis or chorionic villus sampling (CVS). The main disadvantage of this type of genetic testing is that the woman would have no alternative but to choose to terminate her pregnancy in case a genetic disease was detected, given that she has to be already pregnant for these techniques to be carried out.
Alternatively, prospective parents can decide not to use their oocytes and/or sperms to avoid the transmission of genetic diseases to their offspring. In such case, donor gametes would be used.
Can embryo biopsy cause abnormalities in the embryo?
Since this is a very early stage of embryonic development, the embryo will compensate for the absence of the extracted cell and continue to multiply naturally. Therefore, performing PGD on the embryo does not imply any alteration in its genetic endowment.
However, this technique involves the manipulation of the embryo, which may affect its ability to evolve. This is the reason why PGD is generally recommended only in the necessary cases and not in a generalized way.
Recommended reading
As we have said, PGD is a complementary technique that is carried out during in vitro fertilization. If you want to know what this treatment consists of, you can enter the following post: What is In Vitro Fertilization (IVF)? - Process, Cost & Success Rates.
For more detailed information on PGD results and probability of success, you can continue reading in the following article: Understanding the results of Preimplantation Genetic Diagnosis.
We make a great effort to provide you with the highest quality information.
🙏 Please share this article if you liked it. 💜💜 You help us continue!
References
Bick, D.P. y Lau, E.C. (2006). Diagnóstico genético preimplantacional. Pediatr. Clin. N. Am., 53: 559 – 577.
Delhanty, J.D.A. and Handyside, A.H. (1995) The origin of genetic defects in the human and their detection in the preimplantation embryo. Hum. Reprod. Update, 1, 201–215 (View)
Dokras, A., Sargent, I.L., Ross, C. et al. (1990) Trophectoderm biopsy in human blastocysts. Hum. Reprod., 5, 821–825 (View)
Ermanno Greco, Katarzyna Litwicka, Maria Giulia Minas, Elisabetta Cursio , Pier Francesco Greco, Paolo Barillari. Preimplantation Genetic Testing: Where We Are Today. Int J Mol Sci
. 2020 Jun 19;21(12):4381. doi: 10.3390/ijms21124381 (View)
Gleicher, N., Kushnir, V.A. & Barad, D.H. (2014). Preimplantation genetic screening (PGS) still in search of a clinical application: a systematic review. Reproductive Biology and Endocrinology, 12:22 (View)
Ilana Löwy. ART with PGD: Risky heredity and stratified reproduction. Reprod Biomed Soc Online. 2020 Nov 5;11:48-55. doi: 10.1016/j.rbms.2020.09.007 (View)
Manuel Viotti. Preimplantation Genetic Testing for Chromosomal Abnormalities: Aneuploidy, Mosaicism, and Structural Rearrangements. Genes (Basel). 2020 May 29;11(6):602. doi: 10.3390/genes11060602 (View)
Martine De Rycke, Veerle Berckmoes. Preimplantation Genetic Testing for Monogenic Disorders. Genes (Basel). 2020 Jul 31;11(8):871. doi: 10.3390/genes11080871 (View)
Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NE, Arts EG, de Vries JW, Bossuyt PM et al. (2007). In vitro fertilization with preimplantation genetic screening. N Engl J Med; 357: 9– 17 (View)
Moreno, J.M. (2007). Biopsia embrionaria. Aspectos técnicos. ASEBIR, 12: 17-21 (View)
Rodrigo, L.; Rubio, C.; Mateu, E. y Buendía, P., 2014. Capítulo 14: El laboratorio de diagnóstico genético preimplantacional. Instituto Universitario IVI Valencia. Máster en Biotecnología de la Reproducción Humana Asistida. 9ª Edición (2014-2016). 1213-1277
FAQs from users: 'Is it possible to perform PGD on eggs?', 'How many embryos are transferred after PGD?', 'When is PGD recommended?', 'How long do PGD results take?', 'To what extent is the application of PGD important in women who decide to become mothers at an advanced age?', 'What is the present and future of the DGP?', 'When is PGD recommended?', 'When is it advisable to do IVF with PGD or Genetic IVF?', 'What are the main risks of PGD?', 'Why is PGD indicated for women 40 years of age or older?', 'What factors are involved in the success of IVF with PGD?', 'What do statistics show with PGD?', 'Is sex selection possible with PGD?', 'What are the benefits of IVF with PGD?', 'Can autism be prevented through preimplantation genetic diagnosis?', 'Are there any disadvantages of IVF with PGD?', 'How many embryos are necessary for PGD?', 'Would an amnio test become unnecessary if PGD is done?', 'What are the alternatives if IVF with PGD fails to produce healthy embryos?', 'Which genetic diseases are most commonly analyzed through PGD?', 'Is there any alternative to PGD to prevent the transmission of genetic diseases?' and 'Can embryo biopsy cause abnormalities in the embryo?'.
Authors and contributors
More information about Cristina Algarra Goosman









Hi,
I’m from the US, how are you? You see, I’d like to know what are the steps to follow before doing a PGD and how much is it. I’m a carrier of Adrenoleukodystrophy linked to the chromosome X… How can I prevent my baby from developing it?
Thnx
Hello,
The steps to follow before taking the decision of undergoing PGD or not are more or less—depending on each clinic— are as follows:
1. Firstly, you have to undergo IVF, which previous steps are 1) ovarian stimulation; 2) follicular puncture; and 3) embryo culture.
2. Secondly, the embryos are analyzed in the IVF laboratory.
3. Thirdly, only those embryos observed to be healthy are selected for the embryo transfer.
For further info, my advice is that you complete our Form in order to get estimates from different US fertility clinics or check our Clinic Directory out.
Regards