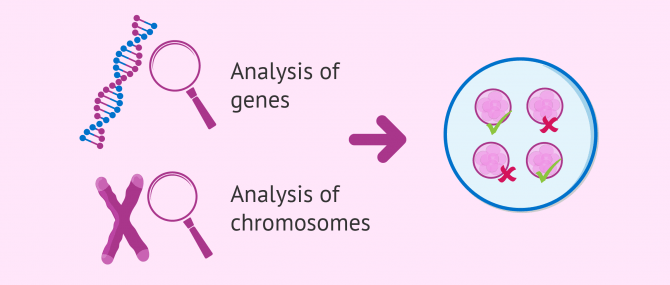
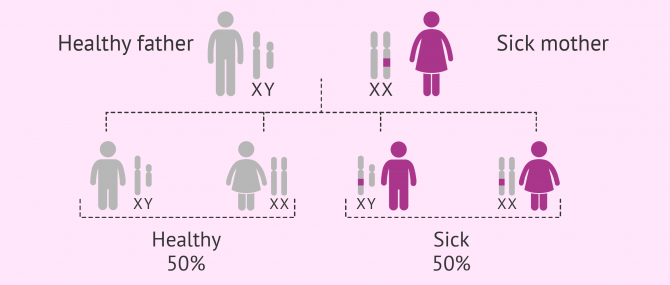PGD or Preimplantation Genetic Diagnosis is a diagnostic technique used in Assisted Reproduction to ensure that embryos are free of genetic abnormalities, including genetic diseases and chromosomal disorders.
PGD is used as an intermediate step in the IVF process, namely when the embryos have been in culture for 3 to 5 days, the stage of embryo development at which we can conduct a blastomere biopsy.
A genetic testing of embryos is recommended, on the one hand, when there exists risk of transmitting a hereditary condition in one or both parents. On the other hand, when both poor quality eggs and sperm are used to create the embryos, as it can lead to an accumulation of DNA mutations.
Provided below is an index with the 10 points we are going to expand on in this article.
- 1.
- 2.
- 2.1.
- 2.2.
- 2.3.
- 2.4.
- 2.5.
- 3.
- 3.1.
- 3.2.
- 4.
- 4.1.
- 4.2.
- 4.3.
- 4.4.
- 4.5.
- 4.6.
- 5.
- 6.
- 7.
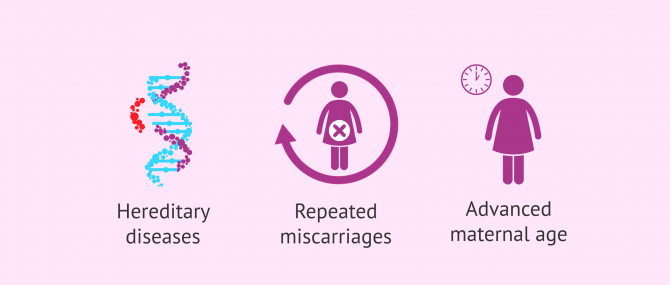
Indications
PGD is a genetic testing of embryos and, as such, it allows us to detect the presence of DNA abnormalities that could lead to miscarriage or the birth of a sick child.
In most cases, the diseases PGD tests for are hereditary, that is to say, they can be transmitted from parents to children. Thus, if one member of the couple has a genetic alteration, or they know that one or both are carriers, they can have healthy children thanks to this method.
Other anomalies, conversely, occur de novo in the resulting embryos after fertilization, without prior history of genetic diseases. In these cases, the main indication for a PGD is having a history of recurrent miscarriages, or in women of advanced age.
During a procedure of IVF with PGD, once the patients have gotten the results of the report, those embryos carrying a genetic abnormality are dismissed. In other words, only healthy embryos, which is to say, embryos that are free of mutations, are transferred back to the womb of the intended mother, or cryopreserved for later use.
Preimplantation Genetic Diagnosis is a controversial technique in several countries. However, it it important to remark that thousands of healthy children have been born worldwide thanks to it.
To perform a PGD, one should undergo IVF as the main treatment. If you are looking for a clinic to get started, we recommend that you generate your individual Fertility Report now. It is a useful, simple tool that, in just 3 steps, will give you a list of the clinics that have passed our rigorous selection process. You will receive an email in your inbox with a report that contains tips and recommendations to get started.
Last but not least, it should be noted that DNA alterations in human beings can be classified into:
- Genetic disorders
- they affect one or various genes.
- Chromosomal disorders
- they affect one or various chromosomes.
According to Antonio Alcaide Raya, BSc, MSc, PhD, Senior Clinical Embryologist and expert in genetic testing:
Today, PGD is available for couples who cannot conceive after various IVF failed attempts with good quality embryos, couples with recurrent miscarriages, when the intended mother is 38 or over, or when one or both parents are carriers or suffer from a genetic disease that could be transmitted to offspring.
Throughout this post, you will have the chance to learn about each one of these types, and we will give you examples of the most common diseases and disorders detectable with PGD.
Genetic diseases
Genetic diseases are caused by genome mutations in the sequence of one gene (monogenic disorders) or several genes (polygenic disorders).
Moreover, when the mutation is present on the reproductive cells (i.e. egg and sperm cells), the disease can be transmitted to offspring.
The chances of transmitting a genetic disease depends on the type of inheritance of each. For this reason, genetic diseases can be classified into the following groups:
Autosomal dominant
Abnormalities that affect non-sexual chromosomes. The affected individual will have the disease, since he or she will inherit a single copy of the faulty gene from one of the parents, who has the disease as well.
The likelihood of passing a genetic disease of this kind from a sick father to his children is 50 percent.
Find the most frequent autosomal dominant disorders in the following list:
- Achondroplasia
- Spinocerebellar ataxia SCA1 y SCA3
- Charcot-Marie-Tooth
- Facioscapulohumeral muscular dystrophy (FSHD)
- Myotonic dystrophy (Steinert)
- Albers-Schönberg disease, or autosomal dominant osteopetrosis, type II (ADO II)
- Tuberous sclerosis type 1
- Tuberous sclerosis type 2
- Hereditary multiple exostoses
- Huntington
- Multiple endocrine neoplasia 1 and 2
- Neurofibromatosis type 1 and 2
- Osteogenesis or osteogenesis imperfecta (OI)
- Hereditary spastic paraplegia (HSP), also called familial spastic paraparesis (FSP)
- Polycystic kidney disease (PKD) linked to the PKD1 region
- Polycystic kidney disease (PKD) linked to the PKD2 region
- Familial adenomatous polyposis (FAP)
- Retinitis pigmentosa
- Lynch syndrome: hereditary non-polyposis colorectal cancer (HNPCC)
- Marfan syndrome
- Noonan syndrome
- Von Hippel-Lindau syndrome
Due to their degree of severity and the high likelihood of transmission to offspring, PGD prior to embryo transfer is strongly recommended for intended parents.
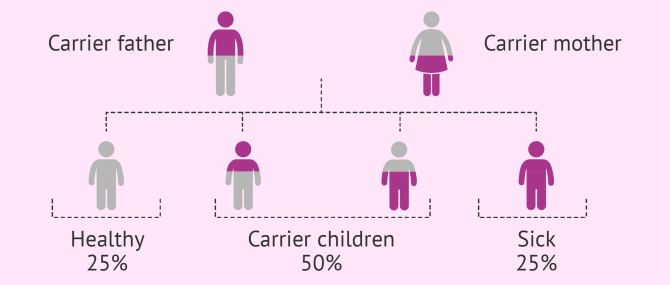
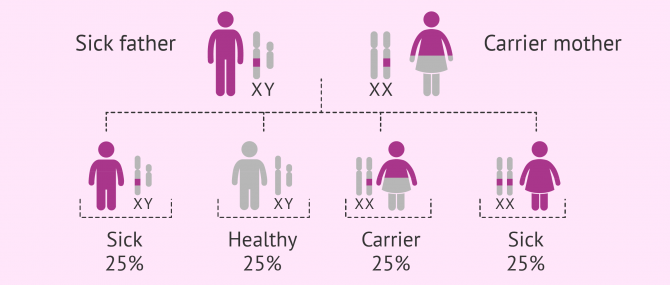
Autosomal recessive
As in the case of autosomal dominant diseases, this group is composed of diseases that affect non-sexual chromosomes. The difference is that the person affected inherits both copies of the defective gen, one from the father and the other from the mother.
If offspring inherited a normal copy and an abnormal one, they would be just carriers of the disease. Carriers do not present symptoms, but they can pass it to future offspring.
The probability of having a sick child with both parents being carriers is 25%, whilst there is a 50% chance for him or her to be a carrier, and 25% of being totally healthy.
Find the most frequent autosomal dominant disorders in the following list:
- Glutaric acidemia type 1
- Propionic acidemia A
- Propionic acidemia B
- Fanconi anemia
- Friedreich ataxia
- Spinal muscular atrophy (SMA)
- α-Thalassemia
- β-Thalassemia
- Disorders of glycosylation (CDG1A)
- Steroid 21-hydroxylase deficiency
- L-CHAD deficiency
- Hidrotic ectodermal dysplasia (Clouston Syndrome)
- Gaucher disease
- Cystic Fibrosis (CF)
- Gangliosidosis
- Severe combined immunodeficiency (SCID)
- Metachromatic leukodystrophy
- Familial haemophagocytic lymphohistiocytosis
- Mucopolysaccharidosis IIIA (San Filippo A)
- Malignant infantile osteopetrosis
- Autosomal recessive polycystic kidney disease (ARPKD)
- Hereditary non-syndromic sensorineural hearing loss
- Tyrosinemia type 1
X-linked dominant inheritance
Mutations that affect the genes on the X chromosome. Since these diseases have a dominant inheritance, they can develop in both males and females.
Sick women have a 50% chance of transmitting the disease to all their sons and daughters. Affected males, however, will pass it to their daughters only, whilst male children will be healthy.
This kind of inheritance pattern occurs rarely. However, the following are some examples of X-linked dominant diseases:
- Pigmentary incontinence
- Hypophosphatemic rickets
- Fabry syndrome
- Rett syndrome
The frequency of this group of disorders is greater in women than in men due to the inheritance pattern. Nonetheless, the severity of the symptoms associated is higher in males, since they have one copy of the X chromosome only.
X-linked recessive inheritance
This group includes mutations that affect the genes on the X chromosome.
Since they have a recessive inheritance, it is necessary for the woman to inherit the defective copies of both parents to be sick. If just one copy is inherited, the affected woman will be just a carrier of the disease.
On the other hand, since males only have one copy of the X chromosome, they will develop the disease in all cases.
The following are some examples of X-linked recessive diseases:
- Adrenoleukodystrophy, also known as X-linked adrenoleukodystrophy, ALD
- Ornithine transcarbamylase deficiency
- Norrie disease
- Hemophilia
- Myotubular myopathy
- Mucopolysaccharidosis I (Hurler syndrome)
- Mucopolysaccharidosis III (Hunter syndrome)
- Alport syndrome
- Fragile X syndrome
- Lesch-Nyhan syndrome
Based on the condition of each progenitor, and the risk of transmitting a disease that is linked to sexual chromosomes, PGD might be recommended or not.
Y-linked inheritance
Mutations that affect chromosomes on the X chromosome. This type of inheritance pattern is known as holandric inheritance.
Given that the Y chromosome can be found in males only, all sons of a male affected will be sick, and could pass it to offspring, too.
Inversely, this type of diseases cannot manifest in females, since the have an XX pair of sex chromosomes.
Finally, one should note that Y-linked genetic diseases occur very rarely. Y chromosome microdeletion (YCM) is an example.
Chromosomal disorders
Chromosomal disorder or abnormalities, also called chromosomopathies, affect the number or structure of chromosomes.
As in the case of genetic disorders, chromosomopathies can be inherited. However, they may occur as the result of a defective meiosis process, which causes abnormalities in the eggs or sperm.
The causes of abnormalities in the meiosis process are varied: being older than 38, cancer treatments, drug abuse, etc.
Certain chromosomal diseases are compatible with life. In these cases, the grade of severity depends on the chromosome that is altered. Others, unfortunately, are incompatible with life and lead to unviable embryos, or embryos that cause recurrent pregnancy loss.
The following are the main types of chromosomal alterations that can be found:
Numerical abnormalities
The total number of chromosomes of human beings is 46—23 from the mother, and 23 from the father.
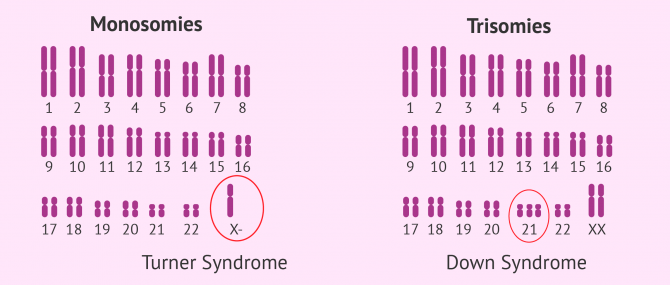
Abnormalities in the number of chromosomes of an individual are known as aneuploidies, and we can be classified into two types:
- Monosomies
- presence of only one chromosome from a pair. It is the case the Turner syndrome, where the affected woman has one X chromosome (karyotype 45,X0).
- Trisomies
- characterized by an additional chromosome. Some examples are the Down syndrome (trisomy 21), Patau syndrome (trisomy 13), Edwards syndrome (trisomy 18), and Klinefelter syndrome (karyotype 47,XXY).
While the chromosomopathies we have just explained above are compatible with life, others like trisomy 15 or trisomy 22 are not.
About sixty percent of miscarriages that occur in the first trimester of pregnancy are due to aneuploidies, as well as 6% of cases of stillbirth.
Structural abnormalities
Structural abnormalities in chromosomes result from breakage and incorrect rejoining of chromosome fragments.
There exist two types:
- Translocations
- transfer of one chromosome segment to another region. We can distinguish between balanced translocations (if the the amount of genetical material does not vary) and unbalanced translocations (if there is additional or missing information). There exists a third type of translocation, the Robertsonian translocation (ROB), in which a pair of acrocentric chromosomes fuse together, which translates into higher risk of trisomy transmission.
- Insertions
- Addition of on chromosome segment to another DNA sequence.
- Inversions
- A chromosome segment is turned upside down (i.e. intrachromosomal rearrangement) and reinserted back into the chromosome.
- Ring chromosomes
- The ends of the chromosome have fused together forming a ring. It has severe consequences, since it is correlated with inactivation.
FAQs from users
Can all diseases be diagnosed with embryo biopsy?
Embryo biopsy provides information about chromosomal endowment or the presence of certain mutations (alterations in genes). This allows us to diagnose chromosomally based diseases early, and some genetically based diseases (those known and legally approved).
However, this technique does not allow the diagnosis of all diseases. Therefore, it is very important to carry out a correct perinatal control of the gestation and of the children born in order to diagnose other types of diseases at an early stage.
Read more
How accurate is PGD for down syndrome?
Preimplantation Genetic Diagnosis or PGD has a margin of error of 1-2%, whether the test indicates that the chromosomes are normal or not. Therefore, although it is very rare, the situation can occur in which a PGT is normal and the embryo is actually affected by trisomy 21 or Down syndrome.
Fundamentally, this error occurs in cases in which there is mosaicism, that is, not all the cells of the embryo have 3 arms of chromosome 21. If the cells obtained in the biopsy are normal, the PGT will be normal. Depending on the proportion of affected and healthy cells, the diagnostic accuracy and the margin of error will depend on this.
Is PGD suitable for women with low ovarian reserve?
Preimplantation genetic diagnosis (PGD) is an assisted reproduction technique that allows the genetic characteristics of embryos to be studied before their transfer to the mother's uterus in order to avoid transmitting genetic diseases or chromosomal alterations.
It is usually recommended when there is a risk of transmitting a genetic disease to the offspring, if one member of the couple has an altered karyotype, in cases of repeated miscarriages, if several unsuccessful IVF cycles have been performed. It can also be indicated in cases of predisposition to cancer of genetic origin and in the case of monogenic diseases (those caused by an alteration in a specific gene).

If we are talking about PGD to confirm that the embryonic karyotype is normal (and not in all cases of diseases or genetic alterations already known), it is usually indicated from 38-39 years of age of the woman, which is when the prevalence of chromosomal alterations in the embryos generated begins to increase significantly. In this case, the probability of having chromosomally altered embryos is very high.
If the patient has a low ovarian reserve it is likely that she will not have many embryos suitable for PGD biopsy, so in each case it will be decided, together with the patient, if it is worth studying them or if this technique can be avoided, depending on her medical history and preferences.
Does PGD / PGS test for Down syndrome?
Yes, either due to advanced maternal age or some kind of abnormality in the karyotype, performing a PGD in these women in order to prevent the birth of a baby with Down syndrome is strongly recommended. This disease distinguishes itself for the presence of three 21 chromosomes instead of having 2, one from the mother and another from the father.
What are the most common diseases leading people to use PGD?
It actually depends on the prevalence of a particular disease. For example, Cystic fibrosis (CF) is the most common genetic disease in the United States and the United Kingdom.
Also, according to Regis College in Greater Boston, Down syndrome, thalassemia, Tay-Sachs disease, and Sickle Cella anemia are common amongst US citizens.
On the other hand, in Spain, Fragile X syndrome, Huntington disease, and muscular dystrophy are the most common diseases leading couples to use PGD.
How are genetic diseases detected in a fetus?
Women who are already pregnant and are at risk of transmitting a genetic disease to offspring, can find out whether the fetus has inherited it or not with an amnio test or chorion biopsy.
Once the result is ready, if it confirms that the fetus has a genetic disease, the woman or couple will have to decide whether they wish to continue with the pregnancy ir terminate it.
Suggested for you
Couples who are at risk of passing a monogenic genetic disorder to their children can use Preimplantation Genetic Diagnosis prior to the embryo transfer. To learn more about this type of diseases, visit: PGD to Detect Monogenic Disorders.
If you wish to delve deeper into the IVF process using PGD step by step, we recommend that you continue reading here: What Is PGD or Preimplantation Genetic Diagnosis?
We make a great effort to provide you with the highest quality information.
🙏 Please share this article if you liked it. 💜💜 You help us continue!
References
Angell, R.R., Xian, J and Keith, J. (1993) Chromosome anomalies in human oocytes in relation to age. Hum. Reprod., 8, 1047–1054.
Buster, J.E. and Carson, S.A. (1989) Genetic diagnosis of the preimplantation embryo. Am. J. Med. Genet., 34, 211–216.
Delhanty, J.D.A. and Handyside, A.H. (1995) The origin of genetic defects in the human and their detection in the preimplantation embryo. Hum. Reprod. Update, 1, 201–215.
Florensa, M. (2011). Diagnóstico genético preimplantacional para enfermedades de aparición tardía. Rev. Asoc. Est. Biol. Rep., Vol. 16, Nº 1, pág. 45-46.
Genetic Alliance; District of Columbia Department of Health. Understanding Genetics: A District of Columbia Guide for Patients and Health Professionals. Washington (DC): Genetic Alliance; 2010 Feb 17. Appendix H, Chromosomal Abnormalities.
Genetics Home Reference (Nov 7, 2018). Inheriting Genetic Conditions. In: Help Me Understand Genetics. Lister Hill National Center for Biomedical Communications U.S. National Library of Medicine National Institutes of Health Department of Health & Human Services.
Harper, J.C. (1996) Preimplantation diagnosis of inherited diseases by embryo biopsy: an update of the world figures. J. Assist. Reprod. Genet., 11, 132–143.
Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NE, Arts EG, de Vries JW, Bossuyt PM et al. (2007). In vitro fertilization with preimplantation genetic screening. N Engl J Med; 357: 9– 17.
Reproducción Asistida ORG. Video: ¿Quién recurre al diagnóstico genético preimplantacional? (When is preimplantation genetic diagnosis used?), by Antonio Alcaide Raya, BSc, MSc, PhD, Aug 2, 2016. [See original video in Spanish].
FAQs from users: 'Can all diseases be diagnosed with embryo biopsy?', 'How accurate is PGD for down syndrome?', 'Is PGD suitable for women with low ovarian reserve?', 'Does PGD / PGS test for Down syndrome?', 'What are the most common diseases leading people to use PGD?' and 'How are genetic diseases detected in a fetus?'.