Embryo freezing is a reproductive technology used to preserve the embryos resulting from an In Vitro Fertilization (IVF) cycle, which could not be transferred in the cycle they were created for.
The most widely used method for embryo cryopreservation today is vitrification, a type of ultra-rapid freezing method that has many advantages over conventional slow freezing methods.
The high survival rates of embryos post-thaw have allowed IVF cycles to be optimized, which reduces the number of attempts necessary to achieve a successful pregnancy significantly.
Provided below is an index with the 9 points we are going to expand on in this article.
- 1.
- 1.1.
- 1.2.
- 2.
- 3.
- 4.
- 5.
- 6.
- 6.1.
- 6.2.
- 6.3.
- 6.4.
- 6.5.
- 7.
- 8.
- 9.
Definition
Unlike conventional freezing, embryo vitrification can be defined as an ultra-rapid freezing process which has the advantage of avoiding ice crystals inside the embryo thanks to the use of cryoprotectants. The damage these crystals cause in its cellular structures may harm the viability of the embryo.
Nowadays, the vitrification technique is used regularly for both egg and embryo cryopreservation. It has become a basic tool in assisted procreation labs. Thanks to it, embryos can be preserved for later use.
Once a vitrification procedure has been performed, they will be stored in specific straws in liquid nitrogen banks at -196°C until they are used. There is no evidence that the time of storage affect their viability and chances of implantation. If the frozen embryo transfer is not performed, patients are consulted every year about what will be embryos used for.
The longest period an embryo has been cryopreserved, allowing the birth of a healthy baby, is 25 years. This is commonly known as snowbabies.
We recommend you visit the following article for more information on this topic: Cryopreservation & Vitrification of Embryos, Sperm & Eggs.
When is it used?
In a cycle of IVF, particularly after ovarian stimulation and egg fertilization, the resulting embryos are cultured for several days.
However, embryos can be cultured for a maximum of 6 days. For this reason, it is necessary to freeze them until we have decided as regards their fate.
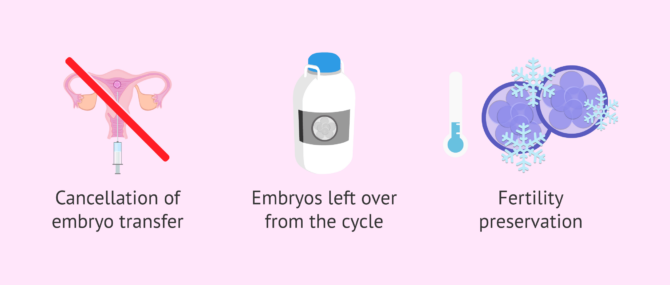
Embryo vitrification also provides a solution in case of, for example:
- Transfer cancellation in the same cycle of stimulation. For instance, due to high risk of developing OHSS or due to inadequate endometrial preparation.
- A fresh embryo transfer has been performed, but there are spare embryos that could be transferred in subsequent cycles in case of negative result or because the patient wants to have more children.
- Fertility preservation. Some couples decide to cryopreserve embryos instead of eggs and sperm separately.
- An embryo biopsy for a PGD/PGS has been performed, but the couple must wait for the results several days.
- In egg donation cycles in case of failure to synchronize the cycles of the donor and the recipient. Vitrification allows for the cryopreservation of either donor eggs or the resulting embryos.
- A woman or couple decide to donate their unused embryos after an IVF cycle to other couples.
Therefore, there are several situations in which it is recommended to vitrify the embryos in order to perform a delayed embryo transfer. It will be essential to evaluate the particular situation of each patient in order to achieve the greatest possible success in the reproductive treatment.
Advantages
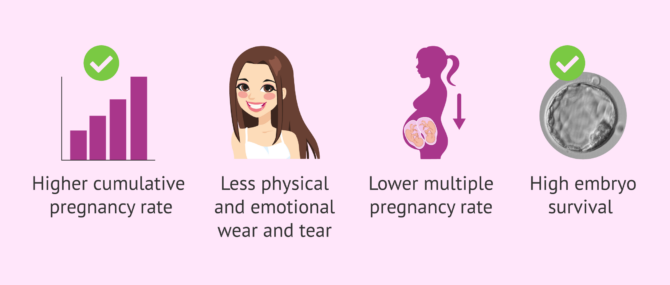
As explained above, embryo vitrification has led to an unprecedented optimization of IVF cycles. In fact, cumulative pregnancy rates (pregnancy rates per egg retrieval in a single cycle of stimulation) have experienced a major increase in the past few years.
The physical and emotional burden for the patient is significantly reduced, since they do not have to administer hormonal medications, including subcutaneous injections, in subsequent cycles. The next IVF cycle will involve a Frozen Embryo Transfer (FET) instead.
Senior Clinical Embryologist Edurne Martínez explains that the embryos are firstly dehydrated before they are immersed in liquid nitrogen. Then, they are rehydrated again and put under a temperature of 37°C, which is the average body temperature of humans.
Martínez qualifies this technique as a highly useful one, since it enables many options for embryo preservation after egg fertilization, either because there are leftover embryos, or because the endometrium or the woman is not ready for the transfer.
Also, embryo vitrification has reduced the multiple pregnancy rates in IVF cycles, given that it allows for the embryos to be transferred one by one.
Finally, it should be noted that, when we compare vitrification with conventional slow freezing technique, the most remarkable advantage of the former over the latter is that the survival rate of embryos is above 90 percent.
Vitrification process step by step
To carry out the vitrification process successfully, the lab staff must be composed of well-versed experts, as it is a challenging procedure that must be done in short time intervals.
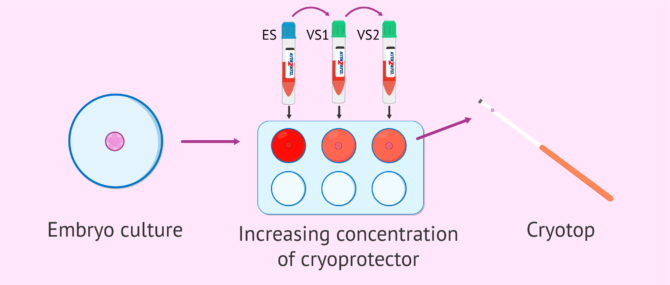
The most common method of vitrification is Cryotop, sold by the brand Kitazato. The following are the general steps followed:
- The embryos are dehydrated, transferring them through different media containing an increasing concentration of cryoprotectants.
- The embryos are gently put onto the support system for being cryopreserved. In the case of Cryotop, this system is a straw.
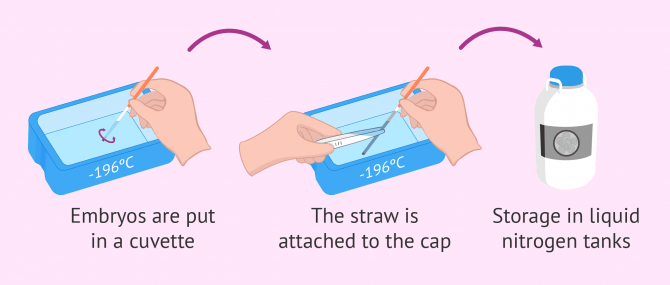
- The straw with the embryos is placed in a small cuvette (container) filled with liquid nitrogen.
- The straw is attached to its cap, taking care that it is not too far from the vapor that comes off the container.
- Finally, the straw filled with the embryos is stored within the liquid nitrogen tank for being cryopreserved.
Throughout the entire process, embryos will drop in just a few seconds, moving from the culture temperature (37 °C) to the temperature of freezing liquid nitrogen (-196 °C).
On the other hand, it is important to keep in mind that embryos must have a minimum quality level to be able to survive the freezing-thawing process. This means that the embryos that do not develop adequately or have signs of degeneration will not be frozen.
Cryoprotectants are a set of molecules that replace the aqueous fluid contained within embryonic cells, which protect the inner structure of embryos from too low temperatures.
Embryo thawing
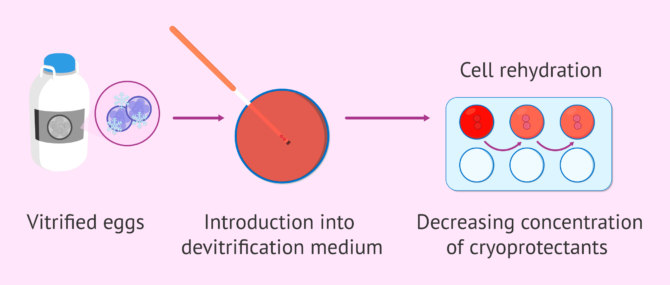
The thawing process of previously frozen embryos is faster and simpler than the freezing one. Simply put, it involves extracting the straw from the liquid nitrogen tank, removing the cap, and putting them directly in a medium at 37 °C.
Aided by a pipette, the embryos are detached from the straw by covering them with a culture medium. Then, the embryos go through several media with decreasing concentrations of cryoprotectants. All this process is done to rehydrate embryonic cells, thereby replacing the cryoprotectants for water.
When carrying out the embryo thawing process, sticking strictly to the times established is crucial. Otherwise, the embryonic cells may be damaged, and the embryo might not be able to survive the process.
Before carrying out a frozen embryo transfer, the embryos must be thawed a couple of hours earlier, and determine whether they have been able to survive or not.
In general, embryo thawing is performed on the day of the embryo transfer, specifically a few hours earlier to check the state of the embryo. Aside from that, the transfer is carried out as if it was a fresh cycle.
Success rates
Fresh IVF cycles have slightly higher success rates than with frozen embryos. But, despite that, frozen embryos can continue their development with normality, attach to the uterus, and most importantly, lead to the birth of a healthy baby.
For example, taking into account embryos that have been genetically analyzed and according to the statistical report of the Spanish Fertility Society (SEF) of 2021, the pregnancy rate by fresh embryo transfer is 46.7%; while the pregnancy rate by frozen embryo transfer is 53.0%.
On the other hand, the delivery rate by fresh embryo transfer is 39.3% and the delivery rate by frozen embryo transfer is 42.0%.
Achieving a pregnancy after a frozen embryo transfer is dependent on these factors:
- The survival rate of embryos post thawing
- Embryo quality and their growth potential
- Endometrial receptivity at the time of the transfer
As explained above, the survival rate of embryos post thaw is high, which proves that they can remain intact. For this to be possible, it is material that the lab has a high-quality vitrification program, and that the staff is careful enough when conducting the procedure.
Cost
Embryo vitrification can cost around $1,000 on average. In Spain, for example, a common destination country for fertility patients, the cost ranges between €350 and €600, depending on the clinic chosen. To this basic fee, we should add the storage fees, since storing the embryos in liquid nitrogen implies a cost for the fertility clinic.
If you are considering cryopreserving your gametes or embryos to have a baby in the future, we recommend that you start by getting a Fertility Report. In 3 simple steps, it will show you a list of clinics that fit your preferences and meet our strict quality criteria. Moreover, you will receive a report via email with useful tips to visit a fertility clinic for the first time.
So, in short, storing the embryos is a service paid by patients, typically on an annual basis, and may add up to $500-$800 per year. Some clinics include the first year of storage in the cost of embryo vitrification.
Moreover, the thawing process and the subsequent frozen embryo transfer can cost $1,000-$2,000 or more. To this, one should add about $400 from the medications to prepare the endometrial lining.
FAQs from users
Is it better to freeze embryos at day 3 or day 5 of development?
Scientific evidence seems to indicate that it is better to transfer embryos at the blastocyst stage, as they will achieve higher gestation rates than embryos at day 3. This increase in implantation rates is due, in part, to a better synchronization between embryo and endometrium, but above all, to a better selection of embryos. Not all day 3 embryos will make it to the blastocyst stage, so if we can discard these embryos by long culture, we will be avoiding useless transfers.
The first studies that were performed on day 3 and 5 embryo freezing seemed to find worse results with embryos at the blastocyst stage. This was because the freezing technique used was slow freezing, which decreased embryo survival to thawing, especially for blastocyst stage embryos. Nowadays, the technique used is vitrification, with embryo survival close to 100%, both for embryos at day 3 and embryos at day 5.
Thus, seeing that blastocysts will give us higher gestation rates than day 3 embryos and that they have a very high survival rate to freezing (using vitrification), it seems clear to deduce that it would be better to freeze day 5 embryos.
It is true that studies have failed to find significant differences in cumulative gestation rate by freezing and transferring embryos at day 3 or 5 of development. What has been shown is that by freezing the embryos at day 5 a smaller number of embryos are frozen and the number of transfers necessary to achieve pregnancy will also be lower.
Does the embryo vitrification technique have any disadvantage?
For many years, freezing of embryos was performed using a “slow-freeze” method. Over the last decade, the technique of vitrification has replaced slow-freeze. The former process involves a rapid freeze method which is very important for oocyte (egg) freezing. Due to the high wáter content of oocytes, the slow freeze method cause crystals to form within the oocytes resulting in suboptimal outcomes.
Vitrification improves all outcomes with embryos and oocytes including survival percentage. Any freezing methods have the disadvantage of the risk of loosing embryos due to post thaw survival, storage tank failure, embryo tolerance for freezing due to patient age, related factor sor the skills of the Embryologist. Currently, there is no known disadvantage unique to the use of vitrification.
Do devitrified embryos have a high survival rate?
The survival rate of the devitrified embryos depends on both the viability of the embryo and tolerance to the process, as well as on compliance with the procedures established in the protocols. Therefore, in order to analyze the survival rate, we must understand what vitrification is.
Furthermore, it should be noted that the success of embryo devitrification does not lie in the survival rate of the embryos after the process. What is really important is to achieve results similar to those achieved when using fresh embryos.
Read more
Can you freeze class C embryos?
Absolutely yes. However, class C embryos present lower implantation rates than class A and B embryos, as their development was inadequate, or they present certain characteristics that may compromise their viability to some extent.
Since the quality of these embryos is poor, in most cases they are cultured for a few days in order to monitor their development. If they grow adequately, they could be cryopreserved. However, if their development stopped, they would be discarded and qualified as unviable.
What are the differences between embryo vitrification and freezing?
Embryo freezing is actually the method used before the vitrification technique was discovered. It was a slow, progressive process that could last up to 2-3 hours. The survival rates were not too high due to the damage caused to the embryos by ice crystals. Although it allowed day 2-3 embryos to be frozen, blastocysts were unable to survive.
Embryo vitrification, conversely, is a ultra rapid procedure that can last just a few minutes, and most importantly, it allows for blastocysts to be cryopreserved as well. This technique is not harmful for the embryos, since it prevents the formation of ice crystals.
You may also enjoy some further information reading this: Cryopreservation & Vitrification of Embryos, Sperm & Eggs.
Recommended reading
As explained above, embryo vitrification is a basic technique nowadays in all IVF labs. Whenever there are unused embryos available, they are cryopreserved for later use. Read more here: What Is the Fate of Unused IVF Embryos?
The transfer of previously vitrified embyros is performed the same as fresh embyros. Nevertheless, if you would like to know more about this procedure then you can continue reading here: Frozen Embryo Transfer.
We make a great effort to provide you with the highest quality information.
🙏 Please share this article if you liked it. 💜💜 You help us continue!
References
Gardner D, Rizk B, Falcone T. Human Assisted Reproductive Technology. Cambridge University Press 2011
José Remohi. Manual práctico de esterilidad y reproducción humana: aspectos clínicos. McGraw-Hill Interamericana de España S.L., 2007
Laura Rienzi, Clarisa Gracia, Roberta Maggiulli, Andrew R LaBarbera, Daniel J Kaser, Filippo M Ubaldi, Sheryl Vanderpoel, Catherine Racowsky. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017 Mar 1;23(2):139-155. doi: 10.1093/humupd/dmw038 (View)
S Canosa, D Cimadomo, A Conforti, R Maggiulli, A Giancani, A Tallarita, F Golia, G Fabozzi, A Vaiarelli, G Gennarelli, A Revelli, F Bongioanni, C Alviggi, F M Ubaldi, L Rienzi; SIERR. The effect of extended cryo-storage following vitrification on embryo competence: a systematic review and meta-analysis (View)
Sociedad Española de la Fertilidad (SEF). Libro Blanco Sociosanitario. La Infertilidad en España Situación Actual y Perspectivas. Imago Concept & Image Development 2011.
Sociedad Española de Fertilidad. Registro Nacional de Actividad 2021-Registro SEF. (View)
Z. BenRafael, Neri Laufer, Shlomo Mashiach, Joseph G. Advances in Assisted Reproductive Technologies Schenker Springer Science & Business Media, 6 dic. 2012.
Zsolt Peter Nagy, Daniel Shapiro, Ching-Chien Chang. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril. 2020 Feb;113(2):241-247. doi: 10.1016/j.fertnstert.2019.12.009 (View)
FAQs from users: 'Is it better to freeze embryos at day 3 or day 5 of development?', 'Does the embryo vitrification technique have any disadvantage?', 'Do devitrified embryos have a high survival rate?', 'Can you freeze class C embryos?' and 'What are the differences between embryo vitrification and freezing?'.
Authors and contributors

More information about Michelle Lorraine Embleton








Hello. I have got several embryos in storage, frozen. My IVF was sucessful first time and I now have a lovely baby girl.
I understand I can use thes embryos in the future, but I don´t really understand what the rest of the embryos are. My question is… will all the embryos be the same? If I use them in the future will they be a “twin” of my daughter?
Thanks in advance
Hi SmileyJanet
Congratulations on your baby girl and how wonderful for you that your treatment was successful forst time round.
The embryos you have in storage are ones that were not transferred in your previous treatment. After the medication you were given to mature your eggs, several eggs would have been collected. Each of these eggs would then have been fertilized by a different sperm cell. This results in several embryos, which you will now have in storage.
As each of the embryos comes from a diofferent egg and fertilized by a different sperm, they will not be “twins” as such. Rather, any children born from using these embryos in future IVF cycles willl be regular brothers and sisters!
I hope this helps to answer your question.
Best wishes