Empty Follicle Syndrome (EFS) occurs when no eggs are retrieved after follicular puncture. This alteration occurs in a small percentage of women who are stimulated for an assisted reproduction treatment and no eggs are found when the follicles are aspirated.
This syndrome was first described in 1986 by Coulam, and has been known to exist ever since. However, the causes that result in the empty follicle syndrome are unknown.
In this article, we'll see what characterizes EFS and how to detect it, as well as the possible treatments or solutions that exist for this syndrome.
Provided below is an index with the 6 points we are going to expand on in this article.
- 1.
- 1.1.
- 1.2.
- 2.
- 3.
- 3.1.
- 3.2.
- 3.3.
- 3.4.
- 3.5.
- 4.
- 5.
- 6.
Empty Follicle Syndrome
During the ovarian puncture, the gynecologist aspirates the follicles in order to extract the eggs and later retrieve them in the laboratory. However, in patients with EFS, the egg fails to detach from the follicle wall and is not retrieved.
EFS is undetectable during ovarian stimulation, as it is characterized by good follicular growth throughout the monitoring phase and adequate blood estradiol levels. Therefore, this syndrome does not present any symptoms until the time of puncture.
The causes of EFS can be classified into two main groups according to their origin: due to a failure in the administration of the treatment medication or due to intrinsic patient causes.
False Empty Follicle Syndrome
False EFS is due to errors or abnormalities in the medications administered for ovarian stimulation.
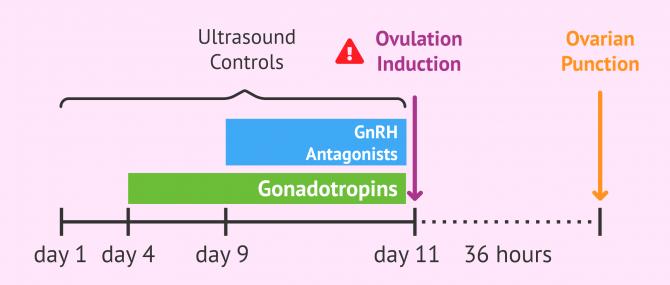
When the follicles have reached an optimal diameter during the stimulation phase, the woman is prepared for the puncture. For this purpose, the woman is administered hCG, also known as trigger, which promotes:
- Maturation of the egg, necessary so that the fertilization of the egg is possible.
- The release of the egg from the follicule wall, as it produces changes in the structure of the connective tissue that makes it more loose.
- Ovulation, which is the release of the egg into the female reproductive tract, it usually occurs about 40 hours after the triggeradministration. However, this event is not desirable and is to be avoided at all costs.
This is why the programming of the puncture is attempted 36 hours after the injection when it's estimated that the egg has matured, but ovulation has not occurred yet.
Errors in time, day, or type of administration of the trigger can cause problems with the necessary time (35-36 hours) for the maturation of the eggs, and therefore, eggs would not be obtained in the ovarian puncture. Misadministration of this drug may also result in insufficient hormone levels to trigger egg maturation.
Other possible errors that could lead to Empty Follicle Syndrome are abnormalities in the trigger medication itself, since it may be outdated or have a decreased effectivity, so it would produce less effect in the patient's body because its depleted active ingredient.
True Empty Follicle Syndrome
True Empty Follicle Syndrome is when the stimulation protocol and trigger administration, follicular growth, and estradiol levels have performed correctly throughout the cycle, yet no eggs have been retrieved from the follicles absorbed by an ovarian puncture.
Another characteristic that makes us think that we are dealing with a case of true EFS is that patients usually continue to respond in the same way in subsequent cycles.
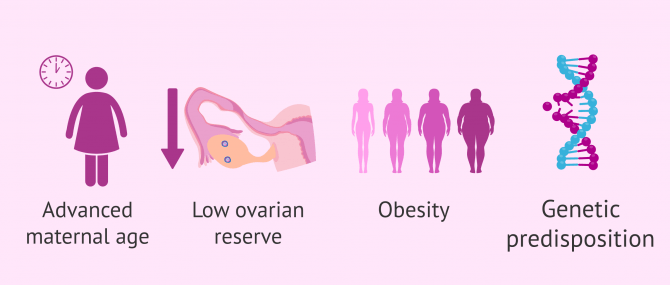
Although the cause that provokes this syndrome in patients is unknown, it has been observed that it affects more frequently women over 40 years old, with low ovarian reserve or obesity. Therefore, this alteration has been related to the aging of the ovaries and a malfunction in the growth and maturation of the follicles.
Another possible cause that could be related to EVS can be explained by genetic alterations that could predispose to this syndrome. This would explain the cases of Empty Follicle Syndrome in young people who do not present alterations in hormonal metabolism or other causes that could predispose them to EFS.
Treatment for Empty Follicle Syndrome
Many studies have highlighted this problem as well as the economic expense and moral burden EFS entails in assisted reproduction treatments.
Luckily, it is a rare event that occurs in only 0.5% to 7% of stimulated assisted reproduction treatments.
Those patients with false EFS due to errors in trigger administration will be able to:
- Determine the hCG levels in the blood to know if they are correct or if a new hCG injection is needed.
- Repeat the puncture if hCG administration has been within the last 36 hours.
- Attempt a new cycle of ovarian stimulation, taking extreme precautions in the administration of medication and aspirating the follicles twice in the puncture.
However, only true EFS reaches 1% of these cases and its prognosis is bleak. This is why many clinics prefer to offer the patient an ovodonation cycle as an alternative option after the impossibility of retrieving mature eggs during several stimulation cycles.
IVF with donor eggs is probably the most confusing of all fertility treatments, and oftentimes, a misleading one. Transparency is one of our strict selection criteria when it comes to recommending fertility clinics to our readers. You can create your Fertility Report now to filter clinics based on our selection criteria and get an individual report based on your preferences with answers to your queries and most importantly, to prevent potential frauds.
Other studies have proposed in vitro oocyte maturation (IVM) as an alternative for these patients, since it does not require trigger injection. In this study, 7 women with at least 3 cycles of conventional IVF where no mature eggs were retrieved were analyzed. All of them underwent IVM and 2 women achieved successful pregnancies.
Despite this isolated article that promises to be hopeful for patients with EFS, it should be noted that IVM is an underused technique and its usefulness is not reliable yet. This is because the success rates obtained by this technique are low. This is why very few clinics offer this treatment to their patients.
FAQs from users
Why can an empty puncture occur?
An empty puncture is a situation in which no oocytes are retrieved during an ovarian punction even though there is a correct growth in the follicles. When we encounter this situation we refer to it as Empty Follicle Syndrome.
Some studies show this can occur in up to 7% of patients that undergo IVF, even though the vast majority of cases can be explained by problems in the hormonal treatment (incorrect administration, outdated HCG...). It is estimated that True Empty Follicle Syndrome is only present in 0.02% of patients.
The causes for this Syndrome are unknown but it is thought that alterations in the folliculogenesis (formation of the follicles and ovules) could be the main cause. These alterations can be caused by advanced maternal age, bad ovarian quality, or genetic factors.
This could cause:
- An early degeneration of the oocytes explaining the lack of them in the punction.
- Lack of detachment of the oocyte from the follicle wall. During ovulation, the luteinizing hormone triggers a series of mechanisms that imply the softening of the follicle´s adhesive tissue, allowing the oocyte to detach from the follicle wall.
Another suggestion we can find is the repetition of the cycle with recombinant hCG, luteinizing hormone, or the trigger of ovulation with the liberation of an agonist of the gonadotropin hormone. If after all of these suggestions the problem persists, ovodonation could be an option.
What is Empty Follicle Syndrome due to?
We refer to Empty Follicle Syndrome (EFS) when the patient is administered correctly the last dose of stimulation and no oocytes are found after the puncture. The lack of physiological response to this last injection is unknown. This lack of reaction can be seen more frequently in women 40 or older, with low ovarian reserve or obesity.
In response to this problem, specialists advise starting a new treatment with a different stimulation protocol or suggest ovodonation in more severe cases.
Read more
Can my Assisted Reproduction cycle be ruined if I don´t inject hCG at the indicated time?
Yes, the success of the assisted reproduction treatment can be compromised if the injection of hCG is administered the wrong way or at the wrong time.
This injection is the last one before the ovarian puncture and is administered, 36 hours before the punction. Its administration promotes a high peak in the LH hormone that helps the oocytes mature and release 38 - 40 hours after its administration, the release is undesirable and must be prevented.
An error in the time of the administration can prevent the oocytes to be retrieved, this can be caused by a False Empty Follicle Syndrome or that the patient has already ovulated.
What alternatives exist for patients with True Empty Follicle Syndrome?
If after a couple of ovarian stimulation syndrome cycles no oocytes are retrieved and the patient is given the diagnosis of True Empty Follicle Syndrome, the first option fertility clinics suggest is ovodonation.
Another suggested technique consists of the maturation of the oocytes in vitro, but this technique is hardly used in fertility clinics.
What are the symptoms of Empty Follicle Syndrome?
Empty Follicle Syndrome (EFS) has no symptoms. Therefore it´s frustrating for specialists as well as for patients, as EFS is not diagnosed during the ovarian stimulation phase, it can only be diagnosed after performing the ovarian puncture and not obtaining any oocytes.
However, a series of measures can be taken into account to prevent this in recurring patients: making sure the patient has understood the hormonal treatment administration instructions, increase the dose of hCG, change the stimulation protocols, or suction repeatedly the same follicle.
Recommended readings
As stated above, there are many stimulation protocols that doctors use in their treatments. The physician's role is to study the patient and assign an appropriate stimulation protocol based on her clinical history. If you are about to start an assisted reproduction treatment with ovarian stimulation and want to know more about this procedure, you can continue reading our article: Ovarian Stimulation Protocols for IVF - Process & Medications Used
Another problem that assisted reproduction experts often encounter in infertility centers is a poor response to hormone treatments. If the stimulation produces few or poor-quality eggs, it can be an impediment to the success of the treatment. This situation occurs more frequently in older women who have delayed childbearing. We recommend the article: Ovarian Stimulation Protocols for IVF - Process & Medications Used, if you want to learn more about this topic.
We make a great effort to provide you with the highest quality information.
🙏 Please share this article if you liked it. 💜💜 You help us continue!
References
Hourvitz A, Maman E, Brengauz M, Machtinger R, Dor J. In vitro maturation for patients with repeated in vitro fertilization failure due to "oocyte maturation abnormalities". Fertil Steril. 2010;94(2):496-501.
Albano C, Grimbizis G, Smitz J, Riethmuller-Winzen H, Reissman T, Van Steirteghem A, Devroey P (1998). The luteal phase of non-supplemental cycles after ovarian superovulation with human menopausal gonadotropin and the gonadotropin-releasing hormone antagonist Cetrorelix. Fertil Steril;70:357 – 359.
Allegra A, Marino A, Coffaro F, Scaglione P, Sammartano F, Rizza G, Volpes A (2007). GnRH antagonist-induced inhibition of the premature LH surge increases pregnancy rates in IUI-stimulated cycles. A prospective randomized trial. Hum Reprod; 22: 101 – 108.
Arici A, Byrd W, Bradshaw K, Kutteh WH, Marshburn P, Carr BR (1994). Evaluation of clomiphene citrate and human chorionic gonadotropin treatment: a prospective, randomized, crossover study during intrauterine insemination cycles. Fertil Steril;61:314 – 318.
Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC (2007). Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod;22:980 – 988.
Balasch J, Ballescà JL, Pimentel C, Creus M, Fàbregues F, Vanrell JA (1994). Late low-dose pure follicle stimulating hormone for ovarian stimulation in intrauterine insemination cycles. Hum Reprod; 9: 1863– 1866
Batista MC, Cartledge TP, Zellmer AW, Nieman LK, Loriaux DL, Merriam GR (1994). The antiprogestin RU486 delays the midcycle gonadotropin surge and ovulation in gonadotropin-releasing hormone-induced cycles. Fertil Steril;62:28 – 34
Beckers NGM, Macklon NS, Eijkemans MJ, Ludwig M, Felderbaum RE, Diedrich K, Bustion S, Loumaye E, Fauser BCJM (2003). Non supplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab; 88:4186 – 4192.
Bhattacharya S, Harrild K, Mollison J, Wordsworth S, Tay CCK, Harrold A, McQueen D, Lyall H, Johnston L, Burrage J et al. (2008). Clomiphene citrate or unstimulated intrauterine insemination compared with expectant management for unexplained infertility: pragmatic randomised controlled trial. BMJ;337:716 – 723.
Braat DD, Schoemaker J (1991). Endocrinology of gonadotropin-releasing hormone induced cycles in hypothalamic amenorrhea: the role of the pulse dose. Fertil Steril;56:1054– 1059.
Cantineau AE, Cohlen BJ. Dutch IUI Study Group (2007), The prevalence and influence of luteinizing hormone surges in stimulated cycles combined with intrauterine insemination during a prospective cohort study. Fertil Steril;88:107– 112.
de Koning J, Lambalk CB, Helmerhorst FM, Helder MN (2001). Is GnRH self-priming an obligatory feature of the reproductive cycle? Hum Reprod;16:209– 214.
Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R (2002). Effect of diagnosis, age, sperm quality, and number of preovulatory follicles on the outcome of multiple cycles of clomiphene citrate-intrauterine insemination. Fertil Steril;78:1088 – 1095.
Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R (2005). Risk factors for higher order multiple pregnancy and multiple birth after controlled ovarian hyperstimulation; results of 4,062 IUI cycles. Fertil Steril; 83:671– 683.
DiLuigi AJ, Nulsen JC (2007). Effects of gonadotropin-releasing hormone agonists and antagonists on luteal function. Curr Opin Obstet Gynecol; 19:258– 265.
Dubourdieu S, Charbonnel B, D’Acremont MF, Carreau S, Spitz IM, Bouchard P (1994). Effect of administration of a gonadotropin-releasing hormone (GnRH) antagonist (Nal-Glu) during the periovulatory period: the luteinizing hormone surge requires secretion of GnRH. J Clin Endocrinol Metab;78:343 – 347.
Ecochard R, Mathieu C, Royere D, Blache G, Rabilloud M, Czyba JC (2000). A randomized prospective study comparing pregnancy rates after clomiphene citrate and human menopausal gonadotropin before intrauterine insemination. Fertil Steril;73:90 – 93
Fauser BCJM, Devroey P, Macklon NS (2005). Multiple birth resulting from ovarian stimulation for subfertility treatment. Lancet; 365:1807– 1816.
Ghesquiere SL, Castelain EG, Spiessens C, Meuleman CL, D’Hooghe TM. Relationship between follicle number and (multiple) live birth rate after controlled ovarian hyperstimulation and intrauterine insemination. Am J Obstet Gynecol 2007;197:589.e1– 5
Gleicher N, Oleske DM, Tur-Kaspa I, Vidali A, Karande V. Reducing the risk of higher order multiple pregnancy after ovarian stimulation with gonadotropins. N Engl J Med 2000;343:2 – 7
Gomez-Polomares JL, Juliia B, Acevedo-Martin B, Martinez-Burgos M, Hernandez ER, Ricciarelli E (2005). Timing ovulation for intrauterine insemination with a GnRH antagonist. Hum Reprod;20:368 – 372
Guzick DS, Carson SA, Coutifaris C, Overstreet JW, Factor-Litvak P, Steinkampf MP, Hill JA, Mastroianni L, Buster JE, Nakajima ST et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. N Engl J Med 1999;340:177 – 183.
Matorras R, Diaz T, Corcostegui B, Ramon O, Pijoan JI, Rodriguez-Escudero FJ. Ovarian stimulation in intrauterine insemination with donor sperm: a randomized study comparing clomiphene citrate in fixed protocol versus highly purified urinary FSH. Hum Reprod 2002;17:2107 – 2111.
Coroleu B, Devesa M, y Álvarez M. Guía 18. Estimulación ovárica para FIV-ICSI en los ciclos con presunción de baja respuesta. Servicio de Medicina de la Reproducción Departamento de Obstetricia, Ginecología y Reproducción Hospital Universitario Quirón Dexeus, Barcelona. Sociedad Española de Fertilidad (SEF) y Sociedad Española de Ginecología y Obstetricia (SEGO)
Ferraretti AP et al. (2011). ESHRE consensus on definition of poor response to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod; 26: 1616-24.
Rodríguez Gálvez, I., Tocino Díaz, A., Fernández Sánchez, M. Fármacos en la estimulación ovárica: clomifeno, gonadotropinas, análogos GnRH, hCG. En: Unidad 06, Bloque I: Esterilidad femenina. Máster en Reproducción Humana de la Universidad Rey Juan Carlos y el Instituto Valenciano de Infertilidad (IVI).
Nelson SM. Biomarkers of ovarian response: current and future applications. Fertil Steril 2013; 99: 963-9.
Hamdine O. et al. (2015). Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum. Reprod.; 39: 170-8.
Griesinger G. y col. (2006). GnRH-antagonists in ovarian stimulation for IVF in patients with poor response to gonadotropins, polycystic ovary syndrome, and risk of ovarian hyperstimulation: a meta-analysis. Reproductive BioMedicine Online; 13: 628-638.
Edwards, R.G., Lobo, R. and Bouchard, P. (1996) Time to revolutionize ovarian stimulation. Hum. Reprod., 11, 917-919.
Emperaire, J.C. and Ruffle, A. (1991) Triggering ovulation with endogenous LH may prevent ovarian hyperstimulation. Hum. Reprod., 6, 506-510.
Lenton, E. (1993) Natural cycle versus stimulated cycle IVF. /. Assist. Reprod. Genet., 10,406-08
Jacob S., Drudy L., Conroy R. and Harrison R.E (1998): Outcome from consecutive in-vitro fertilization/ intracytoplasmic sperm injection attempts in the final group with urinary gonadotropins and the first group treated with recombinant follicle stimulating hormone. Hum. Reprod. 13: 1783-1787.
Celik H, Bıldırcın D, Güven D, Cetinkaya MB, Alper T, Batuoğlu S. Random anti-Müllerian
hormone predicts ovarian response in women with high baseline follicle-stimulating
hormone levels Anti-Müllerian hormone in poor responders in assisted reproductive
treatment. J Assist Reprod Genet, 2012; 29:797–802.
Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L ESHRE
consensus on the definition of “poor response” to ovarian stimulation for in Vitro
fertilization: the Bologna criteria. Human Reproduction 2011; 26 (7): 1616-1624.
Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002; 8(6): 559-77.
Kably Ambe A, Estevez Gonzalez S, Carballo Mondragon E, Dura´n Monterrosas L. Comparative analysis of pregnancy rate/captured oocytes in an in vitro fertilization program. Ginecol Obstet Mex 2008; 76:256 – 260. Spanish
Lee KH, Kim SH, Jee BC, Kim YJ, Suh CS, Kim KC, Lee WD. Comparison of clinical characteristics between early and late patterns in hospitalized patients with ovarian hyperstimulation syndrome. Fertil Steril 2010; 93:2274 – 2280.
Letterie G, Marshall L, Angle M. The relationship of clinical response, oocyte number, and success in oocyte donor cycles. J Assist Reprod Genet 2005;22:115 – 117.
Meniru GI, Craft IL. Utilization of retrieved oocytes as an index of the efficiency of superovulation strategies for in-vitro fertilization treatment. Hum Reprod 1997;12:2129– 2132.
Molina Hita Ma. del M, Lobo Martinez S, Gonzalez Varea, Montejo Gadea JM, Garijo López E, Cuadrado Mangas C. Correlation between the number of oocytes and the pregnancy rate in IVF-ICSI cycles. Revista Iberoamericana de Fertilidad y Reproducción Humana 2008; 25:153 – 159. Spanish
López-Rioja MJ, Campos-Cañas JA, Recio-López Y, Quiroz-Garza G, Sánchez-González M, Hinojosa Rodríguez K, Laresgoiti Servitje E. Número óptimo de ovocitos: modelo de predicción para fertilización in vitro. Ginecol Obstet Mex. 2017 nov;85(11):735-747.
FAQs from users: 'Why can an empty puncture occur?', 'What is Empty Follicle Syndrome due to?', 'Can my Assisted Reproduction cycle be ruined if I don´t inject hCG at the indicated time?', 'What alternatives exist for patients with True Empty Follicle Syndrome?' and 'What are the symptoms of Empty Follicle Syndrome?'.
Authors and contributors
More information about Cristina Algarra Goosman




I had been taking my medication every day at the same time, around 4 pm, I thought I forgot my specialist gave me a set time to take the last shot before punction, and no eggs were retrieved. I am worried I could have EFS even though I took my medication just a little over the time my specialist told me to. Could that be the cause??
Hello Sofia,
In the administration of the last dose, it´s very important to follow your specialist´s instructions, if the hormones are administered differently, at the time of the ovarian punction, the oocytes may haven´t matured enough to separate from the follicle wall and be retrieved, or the matured oocytes have already been detached, in other words, ovulation has already taken place.
A diagnosis for EFS can´t be given until your provider has tested different factors, I suggest you talk to them and try to establish a different protocol.